More Information
Submitted: 25 July 2019 | Approved: 05 August 2019 | Published: 06 August 2019
How to cite this article: Fonseka S, Subhani B, Alahakoon V, Wijeyaratne CN, Gawarammana IB, et al. Effectiveness of ethinyl oestradiol /cyproterone acetate and ethinyl oestradiol/ desogestrel with or without low-dose metformin on perceived health-related quality of life in hirsute women with polycystic ovary disease: A randomised, double-blind, placebo-controlled study. Arch Psychiatr Ment Health. 2019; 3: 025-031. doi: 10.29328/journal.apmh.1001007
Copyright License: © 2019 Fonseka S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Polycystic ovary disease; Quality of life; Hirsutism; Visual analogue scale; Clinical trial
Effectiveness of ethinyl oestradiol /cyproterone acetate and ethinyl oestradiol/ desogestrel with or without low-dose metformin on perceived health-related quality of life in hirsute women with polycystic ovary disease: A randomised, double-blind, placebo-controlled study
Sanjeewani Fonseka1*, B Subhani2, V Alahakoon3, CN Wijeyaratne4, IB Gawarammana5, NS Kalupahana6, N Ratnatunga7, S Rosairo8 and PVR Kumarasiri9
1Department of Pharmacology, Faculty of Medicine, University of Peradeniya, Sri Lanka
2Temporary Demonstrator, Department of Pharmacology, Faculty of Medicine, University of Peradeniya, Sri Lanka
3Temporary Lecturer, Department of Pharmacology, Faculty of Medicine, University of Peradeniya, Sri Lanka
4Professor, Reproductive Medicine, Department of Obstetrics and Gynaecology, Faculty of Medicine, University of Colombo, Sri Lanka
5Professor, Medicine, Department of Medicine, Faculty of Medicine, University of Peradeniya, Sri Lanka
6Professor, Human Nutrition, Department of Physiology, Faculty of Medicine, University of Peradeniya, Sri Lanka
7Senior Professor, Pathology, Department of Pathology, Faculty of Medicine, University of Peradeniya, Sri Lanka
8Senior Lecturer, Department of Radiology, Faculty of Medicine, University of Peradeniya, Sri Lanka
9Professor, Community Medicine, Faculty of Medicine, University of Peradeniya, Sri Lanka
*Address for Correspondence: Sanjeewani Fonseka, Department of Pharmacology, Faculty of Medicine, University of Peradeniya, Sri Lanka, Tel: 0718074979; Email: [email protected]
Background: Polycystic ovary disease (PCOD) is an endocrine disorder. It leads to menstrual disturbances, infertility, obesity and dermatological manifestations such as hirsutism and acne which leads to impaired health-related quality of life (QOL).
Aims: To evaluate the perceived health related QOL in patients with PCOD treated with ethinyl oestradiol (35µg)/cyproterone acetate (2 mg) (EE/CPA) and ethinyl oestradiol (20 µg)/ desogestrel (0.15mg) (EE/DES) alone and in combination with low-dose metformin.
Methods: A total of 117 patients with PCOD diagnosed according to Rotterdam Consensus Criteria 2003 with a hirsutism score of 8 or more according to modified Ferriman-Gallway Score (mFGS) were randomised to receive one of four drug combinations (arm A – EE/CPA, arm B- EE/DES, arm C- EE/CPA plus metformin, arm D- EE/DES plus metformin). The outcomes assessed were body mass index (BMI), hirsutism (using mFGS) and health-related QOL (Polycystic Ovary Syndrome Health- Related quality of life Questionnaire (PCOSQ) and a Visual Analog Scale (VAS) score) at baseline and 12 months after treatment.
Results: PCOSQ score in relation to the hirsutism, emotions, menstruation, obesity, infertility and VAS score in relation to hirsutism and obesity had improved at the end of 12 months (p< 0.001) in all treatment arms. There was no difference between treatment arms in all measured outcomes at baseline and at the end of 12 months.
Conclusion: Treatment with EE/CPA and EE/DES is associated with an improvement in perceived QOL in patients with PCOD. The addition of low-dose metformin did not have a significant benefit.
Polycystic ovary disease (PCOD) is a multisystem disease, and is the commonest endocrinological disease among women of the reproductive age group [1]. It is associated with biochemical and hormonal disturbances leading to adverse cosmetic, reproductive, metabolic, and psychological consequences [2]. A single community study conducted in Sri Lanka, showed that the prevalence of PCOD of a semi-urban population of women is 6.3% [3].
Health-related QOL is defined as the functional effect of a clinical condition, and its treatments upon a patient, which is subjective and multidimensional, including physical function, psychological state, and social interactions. PCOD can impact the QOL of most women leading to psychosocial detriments. The psychological impact and the patients’ mental wellbeing are often-overlooked and inadequately managed in PCOD due to the lack of attention given to it [4].
The cosmetic impact and its associated psychological distress due to hirsutism and obesity, as well as the social tensions associated with infertility can be significant [5-7]. The psychological distress was high in women with hirsutism when compared to women without hirsutism according to some studies whereas others documented significant distress associated with obesity [7,8]. They often suffer from minor to major psychological problems such as, anxiety, body dissatisfaction, eating disorders, diminished sexual satisfaction, depression and lowered health-related QOL. Low self-esteem, decreased social activity and less romantic contentment were reported in women with PCOD [9,10].
The QOL may be objectively assessed by the use questionnaires such as Polycystic Ovary Syndrome Health-Related Quality of life Questionnaire (PCOSQ), 36-Item Short Form Health Survey (SF-36) and the Visual Analogue Scale (VAS) [11,12]. PCOSQ is a reliable instrument for measuring the health-related QOL in women with PCOD [13]. It has been used previously in clinical trials to assess the QOL following treatment [14]. It contains 26 items: emotions (eight items), hirsutism (five items), weight problems (five items), infertility problems (four items), and menstrual problems (four items) [15]. Each question in the PCOSQ is associated with a 7‐point Likert scale in which seven represents optimal function and one represents the poorest function. VAS is a graded linear scale used to assess the patients’ QOL in relation to each of the affected problems such obesity, acne, emotion etc.
A reduced total PCOSQ score in PCOD is associated with sexual dissatisfaction, depression, anxiety, aggression, infertility, weight difficulties, menstrual irregularities and poor interpersonal functioning [16]. Hirsutism, acne and obesity have aesthetic implications. Infertility can lead to various social issues and marital problems particularly in South Asian communities. Most patients with PCOD have difficulty in coming to terms with the disease [17].
Pharmacological treatment with hormonal therapy such as ethinyl oestradiol, desogestrel; antiandrogen therapy such as cyproterone acetate as well as metformin along with life style measures have been shown to have an impact on the improvement in obesity, menstruation, infertility, hirsutism and the emotional state of women with PCOD [5,18-20]. This in turn may contribute towards a better QOL. Thus, we hypothesise that optimal treatment may enhance the health related-QOL in women with PCOD.
The information pertaining to QOL of patients with PCOD and its improvement with treatment was obtained from a larger trial to assess the effectiveness of ethinyl oestradiol (35µg) /cyproterone acetate (2mg) (EE/CPA) and ethinyl oestradiol (20µg)/desogestrel (0.15mg) (EE/DES) alone and in combination with low-dose metformin. This was a single centre, double-blind, placebo-controlled, multi-arm-parallel group study conducted at the Department of Pharmacology, Faculty of Medicine, University of Peradeniya, Sri Lanka. The patients were recruited from the Gynaecology and General Medical Clinics at the Teaching Hospital Kandy and Teaching Hospital Peradeniya which are tertiary referral centres located in the central province in Sri Lanka. Once the patients were recruited from the clinics of the above-mentioned hospitals, they were informed to attend the out-patient clinic at the Department of Pharmacology where they were followed up subsequently during the entire duration of the study. Prior to recruitment of patients all the patients were screened to detect probable contraindications for the medication used in the study. The study population was composed of adult female patients aged 18 – 40 years, diagnosed to have PCOD according to the Rotterdam consensus conference criteria 2003 and a modified Ferriman-Gallway score (mFGS) of 8 or more. Patients who were unable to give consent and having contraindications for the treatment were excluded from the study. Informed written consent was obtained prior to recruitment of patients to the study which was conducted in accordance with the declaration of Helsinki. Ethical approval was obtained from the Ethics Review Committee, Faculty of Medicine, University of Peradeniya, Sri Lanka; registered at the Sri Lanka Clinical Trials Registry (SLCTR) and the registration number was SLCTR/2015/007. Patient recruitment commenced in April 2015 and the follow up ended after 2 years.
All patients were randomized into 4 study arms. The patients in arm A received ethinyl oestradiol (35 µg)/cyproterone acetate (2 mg) daily, placebo pill equal to metformin daily and placebo pill equal to ethinyl oestradiol (20 µg)/desogestrel (0.15 mg) daily. The patients in arm B received ethinyl oestradiol (20 µg)/desogestrel (0.15 mg) daily, placebo pill equal to metformin daily and placebo pill equal to ethinyl oestradiol (35 µg)/cyproterone acetate (2 mg) daily. The patients in arm C received metformin 500 mg daily, ethinyl oestradiol (35 µg)/cyproterone acetate (2 mg) daily and placebo pill equal to ethinyl oestradiol (20 µg)/desogestrel (0.15 mg) daily. The patients in arm D received metformin 500 mg daily, ethinyl oestradiol (20 µg)/desogestrel (0.15 mg) daily and placebo pill equal to ethinyl oestradiol (35 µg)/cyproterone acetate (2 mg) daily.
The objective was to evaluate the change of perceived health-related QOL in patients with PCOD treated with ethinyl oestradiol (35 microg/cyproterone acetate (2 mg) (EE/CPA) and ethinyl oestradiol (20 microg)/desogestrel (0.15mg) (EE/DES) alone and in combination with low-dose metformin (500mg daily). All patients were advised on life style modifications such as a healthy diet and exercise to derive maximum benefit from the treatment. We hypothesised non-inferiority of EE/CPA and EE/DES alone and combination with low-dose metformin in the improvement of health-related QOL in patients with PCOD.
The QOL was assessed by the use of Polycystic Ovary Syndrome Health-Related QOL Questionnaire (PCOSQ). PCOSQ is a reliable instrument for measuring the health-related QOL in women with PCOS [13]. It contains 26 items: emotions (eight items), hirsutism (five items), weight problems (five items), infertility problems (four items), and menstrual problems (four items) [15]. Each question in the PCOSQ is associated with a 7‐point Likert scale in which seven represents optimal function and one represents the poorest function. It has been validated for its reliability as well as the test-retest reliability of the test and internal consistency of the questionnaire. It has been shown to be a multi-dimensional and dynamic tool that measures physical, psychological and social aspects of the disease [15,21].
One shortcoming of PCOSQ is that they do not include acne as one of the markers of assessment. Self-assessment of hirsutism, obesity, acne and alopecia was carried out by the VAS. A graded linear horizontal scale was used, as it appears to be more reliable and has been found to have a higher patient acceptance [22].
The outcomes of the study were the assessment of the health-related QOL assessed by the PCOSQ and VAS score for acne, obesity, alopecia and hirsutism, body mass index (BMI) and hirsutism based on the mFGS at baseline and at the completion of 12 months. The height and weight were measured by a standard technique and the body mass index was calculated. The PCOSQ was carried out as an interviewer administered questionnaire, by the principal investigator at each visit to minimize inter-observer variability.
Considering an alpha value of 0.05 and Beta value of 0.80 the sample size was calculated to 25 patients per arm. We were able to recruit a total of 117 patients with approximately 25-30 patients per arm.
A random number sequence was generated by using an online random number generator (https//www.random.org accessed on 2015-09-13 at 05:02:58 UTC). Thereafter, according to the list generated by random number generator, a piece of paper with one of the letters A, B, C or D was printed, placed inside each envelope, indicating each study arm. The piece of paper was covered with aluminium foil in order to prevent the letter being visible through the envelope and sealed.
When a participant was recruited, the patient’s name, address, telephone number, trial number and file number were stated on the envelope. The envelope was opened and the treatment was started according to the letter on the envelope. All envelopes were kept locked in a drawer. Neither the patient nor the primary investigator knew the drugs the patient was on. Each patient’s study arm was known only by the pharmacist.
Metformin which is a white coloured, odourless and tasteless tablet purchased from the State Pharmaceuticals Manufacturing Corporation, Sri Lanka was used. EE/CPA (Diane® - 35, Schering AG, Berlin, Germany) and EE/DES (Femilon™, Infar (India) Limited) tablets of different colours were obtained. The former was yellow and the latter was white, and came in blister packs of 21 tablets. They were removed from commercial packings and placed in resealable bags and given to the patients. All placebo pills were manufactured at the State Pharmaceuticals Manufacturing Corporation, Sri Lanka. The drugs were stored in separate boxes labelled as A, B, C and D and a pharmacist was in charge of handing over the relevant drugs to the patients which was issued for a duration of 3 months. The patients were given a mobile contact number and requested to contact the department of Pharmacology in the case of an adverse event.
Statistical analysis was carried out at the end of 12 months of follow-up with a per-protocol analysis carried out. All patients who were unable to conclude the study were excluded from the final analysis of the data. Analysis was carried out by SPSS software version 22.0. The data was analysed to find the baseline group differences between completed patients, within group differences and group differences from baseline to 12 months. If the sphericity assumption was not met a Greeenhouse-Geisser correction was carried out. Two-way mixed ANOVA model for repeated measures was used for the analysis. Leven’s and Mauchly’s tests were used.
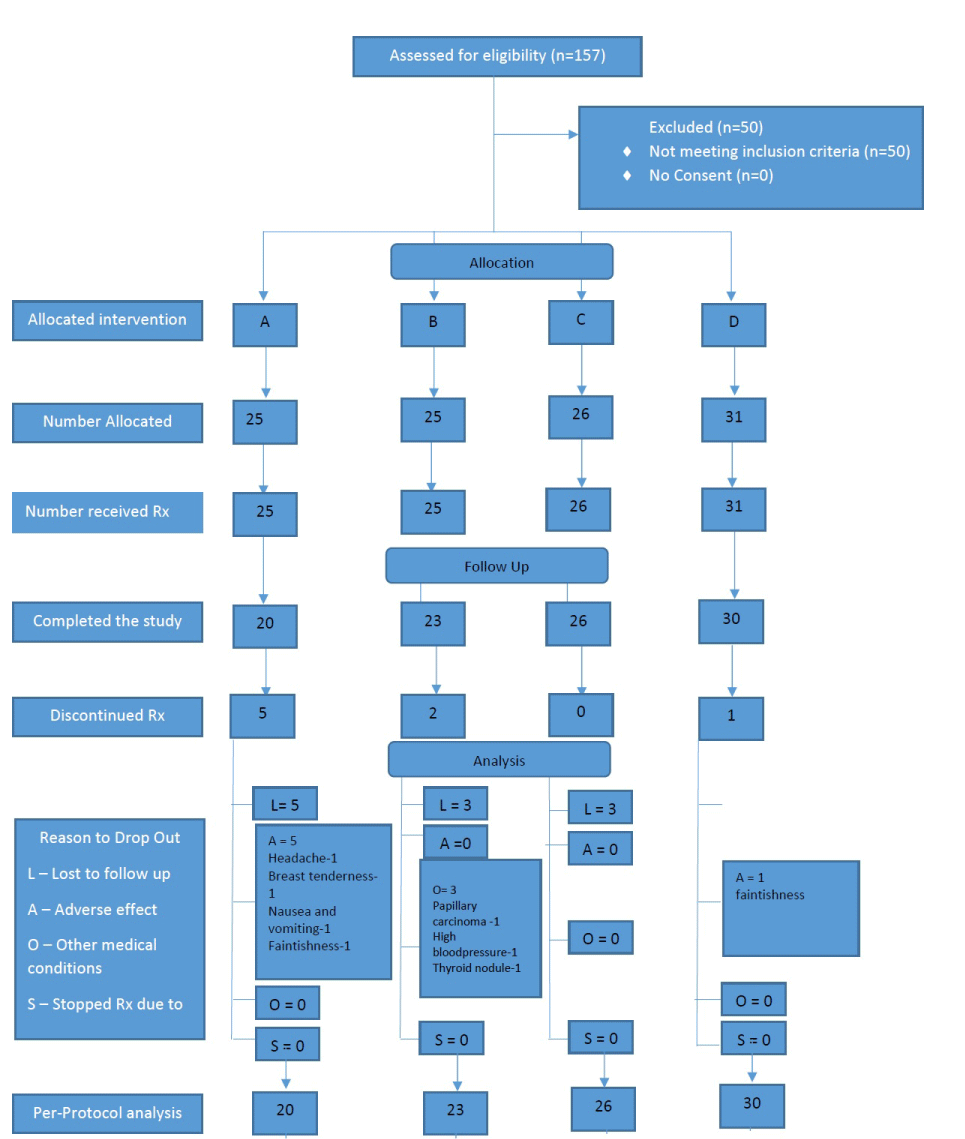
A total of 157 patients were screened for eligibility criteria and 117 patients were recruited for the study as shown in figure 1. In group A, there were 10 dropouts, all of whom had treatment related adverse effects: migraine type headache (3-6 months), joint stiffness (3 months), severe breast tenderness (3 months), vomiting (3 months), a fainting episode (3 months) and 5 were lost to follow up. Group B had 5 dropouts due to incidental diagnosis of papillary carcinoma (3 months), one wanted to conceive (3-6 months) and 3 were lost to follow up. Group C had 3 dropouts who were lost to follow up. Group D had 4 dropouts one due to recurrent fainting episodes (3 months) and 3 were lost to follow up. Patients were recruited from April 2015 for a duration of 2 years and all patients were followed up for a duration of 1 year (12 months). Data from 107 patients who completed the trial was analyzed at the end of the study. The figure 1 shows allocation of patients to each treatment arm. Parameters of QOL, BMI and mFGS of the patients at baseline and at 12 months were as shown in tables 1-4) in the treatment arms A, B, C and D respectively. There was minimal difference in the above parameters at baseline of the patients assigned to the 4 arms.
Figure 1: Shows allocation of patients to each treatment arm.
| Table 1: Baseline and 12 months characteristics of patients in the arm A. | |||
| Baseline | 12 months | p value | |
| PCOSQ score | |||
| Emotions | 30.6 (10.9) | 41.5 (8.8) | 0.001 |
| Hirsutism | 10.5(4.7) | 18.9 (8.9) | 0.001 |
| Obesity | 18.5 (9.3) | 24.8 (8.7) | 0.005 |
| Infertility | 18.5 (8.2) | 25.1 (3.8) | 0.001 |
| Menstruation | 18.3 (6.4) | 23.6 (4.4) | 0.001 |
| Total | 96.3 (28.8) | 133.8 (24.8) | 0.001 |
| Visual analogue scale score | |||
| Acne | 55.5(35.5) | 86.0 (19.6) | 0.003 |
| Obesity | 43.5 (39.9) | 67.0 (29.2) | 0.009 |
| Alopecia | 48.0 (32.7) | 57.0 (33.7) | 0.001 |
| Hirsutism | 19.0 (23.8) | 45.0 (24.4) | 0.003 |
| mFGS | 20.95 (5.5) | 14.25 (8.27) | 0.001 |
| BMI | 28.27 (6.94) | 26.92 (6.68) | 0.003 |
| Table 2: Baseline and 12 months characteristics ofpatients in the arm B | |||
| Baseline | 12months | p value | |
| PCOSQ score | |||
| Emotions | 36.9(8.5) | 44.9(6.0) | 0.002 |
| Hirsutism | 10.7(5.0) | 19.4(7.1) | 0.001 |
| Obesity | 22.3(6.6) | 27.0(7.2) | 0.047 |
| Infertility | 24.1(6.0) | 24.7(3.5) | 0.999 |
| Menstruation | 20.1(5.0) | 23.7(3.8) | 0.013 |
| Total | 114.0(17.5) | 138.9(17.9) | 0.002 |
| Visual analogue scale score | |||
| Acne | 51.7(36.0) | 83.5(24.0) | 0.001 |
| Obesity | 45.7(30.0) | 70.9(19.5) | 0.001 |
| Alopecia | 35.2(35.4) | 67.8(26.5) | 0.001 |
| Hirsutism | 17.8(23.2) | 48.3(21.9) | 0.001 |
| mFGS | 20.00(5.5) | 14.25(8.27) | 0.001 |
| BMI | 26.74(4.88) | 26.09(4.44) | 0.138 |
| Table 3: Baseline and 12 months characteristics ofpatients in the arm C | |||
| Baseline | 12months | p value | |
| PCOSQ score | |||
| Emotions | 31.3(7.6) | 40.9(8.6) | 0.001 |
| Hirsutism | 12.1(6.5) | 19.6(7.8) | 0.001 |
| Obesity | 18.4(9.7) | 23.5(8.1) | 0.015 |
| Infertility | 21.2(6.5) | 23.4(5.2) | 0.999 |
| Menstruation | 18.3(6.3) | 23.6(5.2) | 0.001 |
| Total | 101.4(22.9) | 129(26.9) | 0.037 |
| Visual analogue scale score | |||
| Acne | 44.4(40.7) | 85.8(21.8) | 0.001 |
| Obesity | 33.1(30.4) | 58.1(31.6) | 0.001 |
| Alopecia | 39.6(36.1) | 48.5(25.6) | 0.999 |
| Hirsutism | 16.2(21.9) | 40.8 (23.3) | 0.001 |
| mFGS | 21.08(5.24) | 15.54(5.84) | 0.001 |
| BMI | 27.93(4.28) | 26.78(3.59) | 0.006 |
| Table4: Baseline and 12 months characteristics of patientsin the arm D. | |||
| Baseline | 12months | p value | |
| PCOSQ score | |||
| Emotions | 34.8(8.6) | 42.3(7.7) | 0.001 |
| Hirsutism | 12.7(7.5) | 17.4(6.3) | 0.001 |
| Obesity | 22.5(9.3) | 25.5(7.3) | 0.422 |
| Infertility | 20.8(7.2) | 24.6(3.4) | 0.04 |
| Menstruation | 19.7(5.3) | 24.6(3.2) | 0.001 |
| Total | 135.2(17.1) | 135.2(17.1) | 0.004 |
| Visual analogue scale score | |||
| Acne | 69.8(36.4) | 79. 6 (32.9) | 0.999 |
| Obesity | 47.3(32.3) | 53.2(24.2) | 0.999 |
| Alopecia | 43.8(30.6) | 57.5(30.4) | 0.427 |
| Hirsutism | 24.3(25.0) | 41.4(19.4) | 0.04 |
| mFGS | 18.38(5.53) | 13.72(6.301) | 0.001 |
| BMI | 27.20(4.28) | 26.78(3.59) | 0.227 |
The variation in between the PCOSQ score and VAS score at baseline and at 12 months in arm A (following treatment with EE/CPA without metformin) were as shown in table 1. There was a significant change in the perceived QOL pertaining to emotions, hirsutism, obesity, infertility, menstruation, acne and alopecia in the PCOSQ and VAS scores following the treatment. A significant change was observed in mFGS and BMI compared to the baseline values despite of an absence of metformin.
The variation in between the PCOSQ score and VAS score at baseline and at 12 months in arm B (following treatment with EE/DES without metformin) were as shown in table 2. There was a significant change in the perceived QOL pertaining to emotions, hirsutism, obesity, menstruation, acne and alopecia in the PCOSQ and VAS scores following the treatment but no significant change pertaining to the infertility related scores. A significant change was observed in mFGS compared to the baseline but no significant change in relation to the BMI when compared to the baseline as metformin was not included as part of the treatment but the perceived QOL pertaining to obesity had improved.
The variation in between the PCOSQ score and VAS score at baseline and at 12 months in arm C (following treatment with EE/CPA with metformin) were as shown in table 3. There was a significant change in the perceived QOL pertaining to emotions, hirsutism, obesity, menstruation, acne and alopecia in the PCOSQ and VAS scores following the treatment but no significant change pertaining to the infertility and alopecia related scores. A significant change was observed in mFGS and BMI when compared to the baseline as metformin and an antiandrogen were included as part of the treatment.
The outcomes of the PCOSQ and VAS scores following treatment as well as the differences between the treatment arms are demonstrated in table 5. The total PCOSQ score and PCOSQ scores in relation to hirsutism, menstruation, and emotions significantly improved in all treatment arms irrespective of the type of treatment. PCOSQ score in relation to obesity significantly improved in arms A, B and C even though the BMI had significantly improved only in arms A and C after the intervention. PCOSQ score related to infertility improved in treatment arms A and D. Hirsutism, psychological and menstruation problems in relation to PCOS were equally and significantly reduced in all patients. The mFGS score also significantly reduced in all treatment arms.
| Table 5: Main outcomes of the study in the 4 study arms. | |||||||||||||||
| Main effect of treatment on the outcome | Main effect between the treatment arms on the outcome | Interaction between treatment types | |||||||||||||
| Effect Size (F value) | Error | Degree of freedom | p value | partial ɳ2 | Effect Size (F value) | Error | Degree of freedom | p value | partial ɳ2 | Effect Size (F value) | Error | Degree of freedom | p value | partial ɳ2 | |
| PCOSQ Score | |||||||||||||||
| Emotion | 83.56 | 94 | 1 | <0.001 | 0.471 | 2.7 | 94 | 3 | 0.05 | 0.079 | 0.493 | 94 | 3 | 0.688 | 0.015 |
| Hirsutism | 79.758 | 94 | 1 | <0.001 | 0.459 | 0.289 | 94 | 3 | 0.833 | 0.009 | 0.487 | 94 | 3 | 0.692 | 0.015 |
| Weight | 36.065 | 94 | 1 | <0.001 | 0.227 | 1.568 | 94 | 3 | 0.048 | 0.048 | 0.831 | 94 | 3 | 0.48 | 0.026 |
| Infertility | 21.8 | 94 | 1 | <0.001 | 0.188 | 1.37 | 94 | 3 | 0.257 | 0.042 | 3.064 | 94 | 3 | 0.032 | 0.089 |
| Menstruation | 81.787 | 94 | 1 | <0.001 | 0.465 | 1.37 | 94 | 3 | 0.661 | 0.017 | 0.558 | 94 | 3 | 0.644 | 0.017 |
| Total | 151.378 | 94 | 1 | <0.001 | 0.617 | 2.05 | 94 | 3 | 0.112 | 0.061 | 1.456 | 94 | 3 | 0.232 | 0.044 |
| VAS Score | |||||||||||||||
| Acne | 58.629 | 94 | 1 | <0.001 | 0.384 | 0.751 | 94 | 3 | 0.524 | 0.023 | 4.048 | 94 | 3 | 0.0009 | 0.114 |
| Obesity | 41.736 | 94 | 1 | <0.001 | 0.307 | 1.08 | 94 | 3 | 0.361 | 0.033 | 2.47 | 94 | 3 | 0.067 | 0.0073 |
| Alopecia | 20.649 | 94 | 1 | <0.001 | 0.18 | 0.525 | 94 | 3 | 0.666 | 0.016 | 2.261 | 94 | 3 | 0.086 | 0.067 |
| Hirsutism | 62.186 | 94 | 1 | <0.001 | 0.398 | 0.439 | 94 | 3 | 0.726 | 0.014 | 0.845 | 94 | 3 | 0.473 | 0.026 |
| mFGS | 107.221 | 94 | 1 | <0.001 | 0.533 | 0.819 | 94 | 3 | 0.486 | 0.025 | 0.697 | 94 | 3 | 0.556 | 0.022 |
| BMI | 20.155 | 94 | 1 | <0.001 | 0.177 | 0.284 | 94 | 3 | 0.837 | 0.009 | 1.171 | 94 | 3 | 0.325 | 0.036 |
VAS score in relation to hirsutism significantly improved in all four treatment arms. VAS score in relation to obesity and acne significantly improved in arms A, B, and C and the score in relation to alopecia significantly improved in arms A and B. VAS score in relation to obesity and hirsutism showed an equal improvement according to both methods of assessments. Patients in arm D were not satisfied with the weight loss based on both the PCOSQ and VAS.
There was a significant interaction between the treatment types and time on the health-related QOL on the outcomes of weight, emotion, hirsutism, menstruation but not for infertility and acne. No single arm showed a superior outcome in relation to other arms of treatment, with regard to parameters of health-related QOL.
We attempted a combination of pharmacological treatment in combination with life style modifications, in the treatment of patients with PCOD and assessed the impact of the treatment on the perceived health related QOL of the patients. There is a lack of adequate studies carried out in Sri Lanka, which looked at the perceived QOL of patients with PCOD or the impact on the perceived QOL following treatment. Though we anticipated a difference in the perceived QOL based on PCOSQ and VAS in between the treatment arms in each of the parameters that was assessed, we were unable to detect any differences both at baseline and at the end of the study at the completion of 12 months.
Though there was no significant difference in between the treatment arms, all patients reported an improvement of perceived health related QOL at the end of the study. This may have been contributed by the improved life style modification which has been shown to have a significant positive impact on the QOL of patients with PCOD as well as the peer support gained by being with fellow patients with whom they had formed support groups [23].
Though certain studies have demonstrated that the use of metformin contributes towards improvement of the overall QOL pertaining to psychosocial, emotional and psychosexual health; the patients in our study allocated to both arms C and D did not show a significant improvement compared to the other arms (in relation to menstruation, emotional disturbances, hirsutism and infertility) nor was there a significant improvement in the obesity related perceived QOL score in patients in arm D who received metformin [24,25].
Even though we did not do carry out any intervention in relation to infertility, scores pertaining to the health-related QOL with regard to infertility had improved in some of the arms. The reasons for this may be patients improved general well-being, regular menstruation with hormonal treatment and increased self-confidence with regard to dealing with the disease [5]. There was improvement of the mFGS due to a combination of the hormonal therapy, anti-androgen therapy and insulin sensitizers (metformin) which may have resulted in improved QOL pertaining to hirsutism in all the arms [5,20].
It has been shown that patients with PCOD are mostly distressed by hirsutism, menstrual irregularity and infertility compared to other features [16]. Difficulty in losing weight has been identified as the most distressing symptom in adolescents and young women with PCOD [26]. Another study showed in a cohort of 128 patients, the main concern was shown to be obesity followed by menstrual difficulties, infertility, emotional disturbances and hirsutism [8]. In our study, the patients predominant concern was hirsutism, possibly because we selected women who had hirsutism as a feature of PCOD. The combined effect of exercise, good dietary patterns and peer-support may have contributed to an overall sense of wellbeing. If we had taken a mixed population of PCOS patients, the results could have been different.
The major limitations of the study included lack of a culturally acceptable tool for the assessment of the QOL and inadequacy of the sample size in each group. Most studies which had studied the QOL in patients with PCOD had used much larger samples in order to detect the differences in between the groups. Further our results were based on patient reporting thus self-reporting bias could not be avoided which may account for some of the positive outcomes at the end of the study.
Treatment can improve the psychosocial and emotional situation of PCOD patients. Although at least some of these effects may be related to the reduction of individual clinical symptoms (i.e. weight loss, normalization of menstrual disturbances, improvement of acne). But superiority of one particular modality of treatment could not be demonstrated. There is a need to validate a culturally acceptable tool to assess the health-related QOL in patients with PCOD.
We would like to acknowledge the contributions made by the staff at the department of Pharmacology to carry out this study.
- Group, REAS-Pcw. "Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS)." Hum Reprod. 2004; 19: 41-47. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14711538
- Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, et al. "Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group." Fertil Steril. 2012; 97: 28-38. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22153789
- Kumarapeli V, Seneviratne Rde A, Wijeyaratne CN, Yapa RM, Dodampahala SH. "A simple screening approach for assessing community prevalence and phenotype of polycystic ovary syndrome in a semi-urban population in Sri Lanka." Am J Epidemiol. 2008; 168: 321-328. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18550559
- Misso M, Boyle J, Norman R, Teede H. "Development of evidenced-based guidelines for PCOS and implications for community health." Semin Reprod Med. 2014; 32: 230-240. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24715518
- Vrbíková J, Cibula D. "Combined oral contraceptives in the treatment of polycystic ovary syndrome." Hum Reprod Update. 2005; 11: 277-291.PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15790599
- Clark NM, AJ Podolski, ED Brooks, DR Chizen, RA Pierson, et al. "Prevalence of Polycystic Ovary Syndrome Phenotypes Using Updated Criteria for Polycystic Ovarian Morphology: An Assessment of Over 100 Consecutive Women Self-reporting Features of Polycystic Ovary Syndrome." Reproductive sciences (Thousand Oaks, Calif.) 2014; 21: 1034-1043.PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24520081
- Guidi J, Gambineri A, Zanotti L, Fanelli F, Fava GA. "Psychological aspects of hyperandrogenic states in late adolescent and young women." Clin Endocrinol (Oxf). 2015; 83: 872-878. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25823959
- McCook JG, Reame NE, Thatcher SS. "Health-related quality of life issues in women with polycystic ovary syndrome." J Obstet Gynecol Neonatal Nurs. 2005; 34: 12-20.PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15673641
- Himelein MJ, Thatcher SS. "Polycystic ovary syndrome and mental health: A review." Obstet Gynecol Surv. 2006; 61: 723-732. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17044949
- Bazarganipour F, Ziaei, Montazeri A, Foroozanfard F, Kazemnejad A, et al. "Psychological investigation in patients with polycystic ovary syndrome." Health Qual Life Outcomes. 2013; 11: 141.PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23947827
- Cronin L, Guyatt G, Griffith L, Wong E, Azziz R. "Development of a health-related quality-of-life questionnaire (PCOSQ) for women with polycystic ovary syndrome (PCOS)." J Clin Endocrinol Metab. 1998; 83: 1976-1987. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9626128
- Brady C, Mousa SS, Mousa SA. "Polycystic ovary syndrome and its impact on women's quality of life: More than just an endocrine disorder." Drug, healthcare and patient safety. 2009; 1: 9-15.PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21701605
- Jones GL, Benes K, Clark TL, Denham R, Holder MG, et al. "The Polycystic Ovary Syndrome Health-Related Quality of Life Questionnaire (PCOSQ): a validation." Hum Reprod. 2004; 19: 371-377. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14747184
- Malik-Aslam A, Reaney MD, Speight J. "The Suitability of Polycystic Ovary Syndrome-Specific Questionnaires for Measuring the Impact of PCOS on Quality of Life in Clinical Trials." Value in Health. 2010; 13: 440-446. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20230548
- Cronin L, Guyatt G, Griffith L, Wong E, Azziz R, et al. "Development of a Health-Related Quality-of-Life Questionnaire (PCOSQ) for Women with Polycystic Ovary Syndrome (PCOS)1." The Journal of Clinical Endocrinology & Metabolism. 1998; 83: 1976-1987.PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6053872/
- Kitzinger C and J Willmott. "'The thief of womanhood': women's experience of polycystic ovarian syndrome." Soc Sci Med. 2002; 54: 349-361.
- Jones GL, Palep-Singh M, Ledger WL, Balen AH, Jenkinson C. "Do South Asian women with PCOS have poorer health-related quality of life than Caucasian women with PCOS? A comparative cross-sectional study." Health and Quality of Life Outcomes. 2010; 8: 149. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21171983
- Mastorakos G, Koliopoulos C, Creatsas G. "Androgen and lipid profiles in adolescents with polycystic ovary syndrome who were treated with two forms of combined oral contraceptives." Fertil Steril. 2002; 77: 919-927. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12009344
- Van der Spuy ZM, le Roux PA. "Cyproterone acetate for hirsutism." Cochrane Database Syst Rev. 2003; 4: Cd001125.PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14583927
- Cosma M, BA Swiglo, DN Flynn, DM Kurtz, ML Labella, et al. "Clinical review: Insulin sensitizers for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials." J Clin Endocrinol Metab. 2008; 93: 1135-1142. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18252787
- Colwell HH, SD Mathias, DJ Pasta, JM Henning and JF Steege. "A health-related quality-of-life instrument for symptomatic patients with endometriosis: a validation study." Am J Obstet Gynecol. 1998; 179: 47-55.PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9704764
- Sriwatanakul K, Kelvie W, Lasagna L, Calimlim JF, Weis OF, et al. "Studies with different types of visual analog scales for measurement of pain." Clin Pharmacol Ther. 1983; 34: 234-239. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/6872418
- Moran LJ, Hutchison SK, Norman RJ, Teede HJ. "Lifestyle changes in women with polycystic ovary syndrome." Cochrane Database of Systematic Reviews (3). 2019. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21328294
- Hahn S, Benson S, Elsenbruch S, Pleger K, Tan S, et al. "Metformin treatment of polycystic ovary syndrome improves health-related quality-of-life, emotional distress and sexuality." Human Reproduction. 2006; 21: 1925-1934. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16549423
- Ou HT, Chen PC, Wu MH, Lin CY. "Metformin improved health-related quality of life in ethnic Chinese women with polycystic ovary syndrome." Health and Quality of Life Outcomes. 2016; 14: 119. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27553217
- Trent ME, Rich M, Austin SB, Gordon CM. "Quality of life in adolescent girls with polycystic ovary syndrome." Arch Pediatr Adolesc Med. 2002; 156: 556-560.PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12038887