More Information
Submitted: 30 October 2019 | Approved: 29 November 2019 | Published: 02 December 2019
How to cite this article: Demirbek B, Demirhan O. Microchimerism may be the cause of psychiatric disorders. Arch Psychiatr Ment Health. 2019; 3: 042-046.
DOI: 10.29328/journal.apmh.1001009
Copyright License: © 2019 de Demirbek B, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Microchimerism may be the cause of psychiatric disorders
Bülent Demirbek1 and Osman Demirhan2*
1Department of Psychiatry, Adana City Training and Research Hospital, Adana, Turkey
2Department of Medical Biology and Genetics, Faculty of Medicine, Çukurova University, Adana, Turkey
*Address for Correspondence: Osman Demirhan, Department of Medical Biology and Genetics, Faculty of Medicine, Cukurova University, 01330 Balcalı, Adana, Turkey, Tel: 0506-0229765; Email: [email protected]; [email protected]
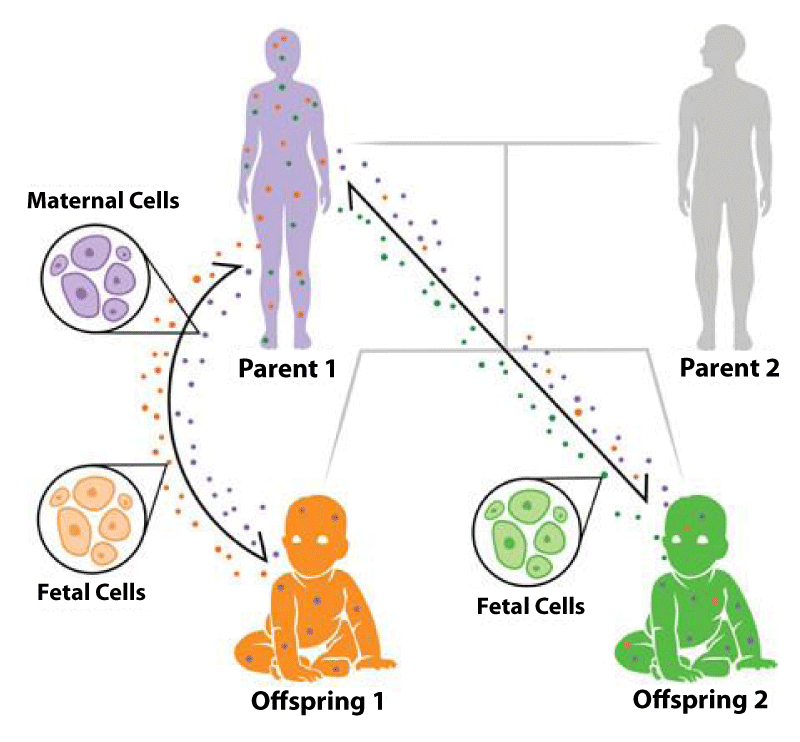
Microchimerism is a bidirectional exchange of fetal and maternal cells during pregnancy (Figure 1). Pregnancy is the most common and natural cause of chimerism, and bi-directional trafficking of hematopoietic cells occurs through the placenta. Therefore, we are all born as microchimera [1,2]. Although there are many unanswered questions it is thought that chimerism has an important role in human health. For many years, the clinical effects of maternal microchimeric cells (MMcCs) in organ repair and cancer therapy have just begun to be understood. While the mission of chimerism is straight forward, the subject is profound. Chimerism carries the potential for disease as well as for health benefits. Recent studies have shown that maternal stress and infections in pregnancy affect fetal neuro development and increased the risk of neurological or psychiatric disorders in the future life of the fetus. This article describes the role of Mc in the etiology of psychotic disorders.
Figure 1: Pedigree of microchimerism. During pregnancy, fetal cells (represented as orange and green circles) traffic into the maternal body, increasing in quantity throughout the gestational period. Likewise, each fetus inherits maternally derived cells (represented as purple circles). It has been predicted that younger siblings could also obtain older siblings’ cells, as depicted with offspring 1 cells (orange) circulating within the younger sibling’s body (offspring 2).
The relationship between microchimerism and psychiatric disorders
Bi-directional cell transfer between mother and fetus during pregnancy is a physiological phenomenon (Figure 1). Therefore, we were all born microchimerically. Today, however it is not known exactly why Mc exists or what its task is. Therefore, the physiological task of Mc is a mysterious and incomplete issue. Mc is also seen as a physiological component of the immune system. Pregnancy-related migratory progenitor fetal cells (FPCs) have been shown to merge with multiple maternal organs. The findings support the likelihood that fetal cells frequently cross the human blood-brain barrier and that Mc in the brain is relatively common. However, the association of these fetal cells (FCs) with the brain, their long-term survival and differentiation are not fully known. However, FPCs were shown to mature and differentiate into neurons in the mother brain. In fetal-maternal Mc, it was shown that FPCs were joined with various regions of the maternal brain until the 7th month after birth and became permanent [3]. In addition to expressing neural stem cell or immature neuron markers, FPCs were expressed mature neuron markers and indicated that they adopted a neuron fate. In addition, neuronal maturation of these cells was observed with increasing axonal dendritic complexity. Thus, FPCs appear to undergo a molecular and morphological maturation program similar to that observed during adult neurogenesis.
The physiopathological state
Each physiological event has a physiopathological state. During the first months of pregnancy, viral infections of the mother is disrupt the physiology. As a matter of fact, maternal cells pass from mother to fetus in the first 4.5 months of pregnancy due to viral infections of mother. In this way, the fetus was protected from viral diseases. Infectious diseases can increase routine cell migration between mother and fetus. The mother’s cell, which passes to protect the fetus, plays a role in the construction of various tissues and organs before returning. There are different interpretations of the functions of these MMcCs that settle in fetal tissues and do not return. It is known that neurodevelopment in human’s results from the interaction of genetic, epigenetic and environmental factors. In mammals, the building blocks of brain development are completed before birth. We think that these non-returning cells were cause physiopathological Mc. Recent findings suggest that there is a relationship between schizophrenia and immune system disorders. The relationship between maternal infection and neurodevelopmental disorders has long been known. Although schizophrenia was considered a syndrome of different biological backgrounds, the inclusion of immune system disorders may be one of the common mechanisms. The strongest evidence for maternal infection increasing risk for a mental disorder in the offspring is the connection between schizophrenia and maternal respiratory infection [4,5]. An increase in the amount of viral infection and schizophrenia was detected in winter and spring. Thus, the risk for schizophrenia in the offspring was increased 3-fold by infection in the second trimester [4]. Numerous animal studies demonstrate that prenatal or early postnatal infections can result in both acute and persistent neurological and behavioral abnormalities in offspring resembling autistic traits or schizophrenia [4,6]. Modeling this risk factor in animals, when influenza infection is induced in pregnant rodents during pregnancy, their offspring Show several behavioral and histological abnormalities consistent with human mental illness [7]. In 1964, the incidence of schizophrenia in the population exposed to rubella epidemic increased from 1% to 20% [8]. Subsequent studies have shown that historical outbreaks such as influenza, measles, mumps, chicken pox and polio are associated with schizophrenia [9]. However, several prospective studies [10,11] revealed a relationship between maternal viral infection and psychiatric disorders in offspring [12,13]. During the months of winter and spring when the incidence of viral infections as well as the incidence of schizophrenia births are high, supporting our hypothesis [14]. How can the above pathogens provide the risks of psychotic disorder? The common response to these pathogens is maternal immunity. Indeed, exposure to environmental pollutants leading to high immune responses such as maternal autoimmune disorders, allergies, asthma and acute stress has been reported to increase the risk of schizophrenia [10,11]. All this evidence points to the important role of neuro-inflammation and immune system in the pathophysiology of schizophrenia [14]. However, there were numerous reports supporting the hypothesis that immune activation is a risk for the onset of schizophrenia in adulthood [15,16]. Schizophrenic clinical observations observed after bone marrow transplantation will significantly enhance our understanding of the importance of the immune system in schizophrenia [17,18].
However, we think that maternal cells can pass on the knowledge acquired through the migration through the mother’s human history to the offspring. Given the capacity of DNA to store 2,5 billion terabytes of information, it should not be difficult to transfer the information acquired throughout human history to the new generation. Thus, the fetal cells pass through the mother’s body and have the opportunity to learn from the source the information acquired in the mother throughout human history. Afterwards, these cells may pass back to the breast tissue and return to the baby with familial information they copy from the mother through breastfeeding. The fate of these cells in offspring has not been adequately described. In a recent study; it was confirmed that maternal cells were detected in the blood and brain of the offspring and that these cells differentiated into both neuron and glia cells in the brain [1]. This shows us the presence of Mc in the brain due to breastfeeding. Thus, the transfer of acquired fear, behavior and psychological gains to the new generation may be guaranteed non-genetic. In addition to fetal brain development, MMcCs can affect postnatal cognitive performance and behavior.
Evidence from research over the past decade shows that the parental environment can have a profound impact on future generations. This supports our thinking; a recent study showing that parent ‘memory’ was inherited between generations of Drosophilla melanogaster flies [19], it was reported that new behavior continued for five generations, not just one generation. Researchers have shown that even though neuronal coded ancestral behavior is not thought to be hereditary for generations, environmentally triggered modifications that of parental experiences, may allow the memory to remain hereditary. Therefore, it is important to examine the impact of parental experiences affecting future generations. There are many human studies that show that parental stress environments affect at least the next generation. Dutch Hunger Winter [II. Close to the end of World War I, the Dutch famine of 1944-1945] and a generation of food deprivation have shown a number of studies showing that offspring and grandchildren may experience permanent metabolic changes. There were some studies showing that there is an increase in anxiety in children of families with paternal post-traumatic stress disorder. There were also some interesting studies in mice and other model systems. All this generally indicates that there is some extra environmental information that is transferred off-chromosomally to children. However, Drosophila melanogaster flies can have highly conserved molecular mechanisms despite human and all animal species, and many things can work similarly.
Recent studies have shown that brain development in response to prenatal stress may change by epigenetic inheritance for several generations, independent of genetic predisposition. Observational studies also show that prenatal adverse environmental effects, such as maternal stress and infections, affect fetal neuro-development and increase the risk of neurological or psychiatric disorders in future life [20-22]. Disruption of neurodevelopmental pathways can affect cognitive and mental disorders as well as affect future brain function and may increase the risk of neurodevelopmental and psychiatric disorders in later life [23]. The adaptation processes of the fetus are very sensitive to negative stresses such as maternal stress and/or maternal infections. In this context, chronic stress conditions affect pregnant women [24]. In addition, such adverse life events may pose a significant threat to maternal health during pregnancy. Excessive stress experienced by the mother during pregnancy can lead to autism spectrum disorder [25], depressive symptoms [26,27], anxiety, personality disorder, eating disorders [28] and attention deficit/hyperactivity disorder [21,29,30]. In addition to high levels of perception of stress, maternal infection during pregnancy may interfere with the neural development of the fetus and may increase the risk of offspring neurological dysfunction, psychiatric disorders, and autism spectrum disorder [31].
Too many maternal cells for to fight infectious diseases that pass to the fetus cannot return to the mother’s body. These cells are maternal leukocytes and lymphocytes somatic cell nature. When suitable agents were used, these cells are “dedifferentiated” and can be transformed into stem cells. Fetal cell medium is a medium where stem cells are dense. We believe that maternal cells passing into fetus are first differentiated into stem cells by dedifferentiating, then differentiated (Redifferentiated) and involved in the construction of tissues and organs. This will cause allo-immunity because the DNAs of the cells involved in the construction of tissue organs are different. Today, we must recognize that maternal microchimeric cells cause autoimmune diseases, and that these cells are the etiologic cause of alloimmune diseases, and should be treated accordingly (also in psychiatric diseases). If the maternal cells that acquire physiopathological features participate in the production of the brain, there are 2 different groups of DNA in the brain. In this case, two different neuromediator-receptor groups will be present in the same brain tissue. The production of each DNA is individual. Chaos physics rules begin to work and the brain’s architecture (configuration) changes as the initial conditions change. In babies born with brain changing architecture, ‘Soft Neurological Symptoms’ were detected [32].
Can the concept of fetal-maternal cell migration explain the full recovery of 1/3 of schizophrenic patients after the first attack?
If these nomadic cells are unable to produce stem cells that can sustain them, schizophrenia will heal after the first episode, since new nomadic cells that are cleared from the schizophrenic brain are not replaced. If the nomadic cells were able to produce stem cells that would allow them to survive, they would produce new nomadic cells instead of being dragged into apoptosis and destroyed. In this case, schizophrenia would have gained chronicity.
Fetö-maternal cell migration can explain the concept of ‘hallucination’. In other words, if there are two different DNAs in a brain tissue, neuromediator-receptor production will be individualized. Even if the dopamine produced by schizophrenic brain cells reaches nomadic cell reptors, it will not be able to bind because the ‘rezonance frequency’ is different. Therefore, nomadic cell receptors will continue to demand dopamine due to dopamine hunger and dopamine will be continuously produced and delivered. Thus, there will be a relative abundance of dopamine in the environment. In this case, dopamine will reach and activate the hearing receptors and the schizophrenic person will hear it as a sound. Vibration frequency, in a study on Drosophila melanogaster flies, the compartment where the hydrogenated apple essence, flies have always found the right. However, when deuterium or tritium was used instead of hydrogen in apple essence, flies did not correctly locate the area of apple essence. Because doteryum or tritium was added to the apple essence, it changed the vibration frequency of the essence because these substances were heavier than hydrogen. We can compare the vibration frequency to the radio-frequency switches that drive our car. In appearance, although all keys and slots are similar, one key cannot start another car because the radiofrequency is different. The resonance frequencies of the neuromodulatory receptor pair produced by each DNA are also different. We think that psychotic behavior seen in this postpartum mother may be seen in this way. In a recent study, we reported that there was significant difference between women with postnatal depression and healthy women. MMc prevalence was significantly greater in women with postnatal depression than control groups [32]. Mc is known to play an etiological role in autoimmune diseases, and may also be the causative factor in the development of schizophrenia and postpartum psychosis [33].
We believe that Mc may be an important alternative explanation to the etiology of psychiatric diseases. Thus, numerous studies demonstrate that prenatal or early postnatal infections can result in both acute and persistent neurological and behavioral abnormalities in offspring resembling autistic traits or schizophrenia. Current studies will provide valuable insights into the effects of the immune system on brain and behavior, and represent an important potential step towards more personalized medicine for patients with schizophrenia.
- Miranda PD, Goulmy E. We are all born as microchimera. Chimerism. 2013; 4: 18-19. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23262442
- Guettier C, Sebagh M, Buard J, Feneux D, Ortin-Serrano M, et al. Male cellmicrochimerism in normal and diseased female livers from fetal life to adulthood. Hepatology. 2005; 42: 35-43. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15962317
- Zeng XX, Tan KH, Yeo A, Sasajala P, Tan X, et al. Pregnancy-associated progenitor cells differentiate and mature into neurons in the maternal brain. Stem Cells Dev. 2010; 19: 1-12. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20707697
- Dubernard G, Aractingi S, Oster M, Rouzier R, Mathieu MC, et al. Breast cancer stroma frequently recruits fetal derived cells during pregnancy. Breast Cancer Res. 2008; 10: R14. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18271969
- Nguyen Huu S, Oster M, Avril MF, Boitier F, Mortier L, et al. Fetal microchimeric cells participate in tumour angiogenesis in melanomas occurring during pregnancy. AmJ Pathol. 2009; 174: 630-637. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19147820
- Gadi VK. Fetal microchimerism and cancer. Cancer Lett. 2009; 276: 8-13. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18845390
- Sawaya HHB, Jimenez SA, Artlett CM. Quantification of fetal microchimeric cells in clinically affected and unaffected skin of patients with systemic sclerosis. Rheumatology. 2004; 43: 965-968. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15199216
- Brown AS, Cohen P, Harkavy-Friedman J, Babulas V, Malaspina D, et al. A.E. Bennett Research Award. Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol Psychiatry. 2001; 49: 473-486. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11257233
- Reisinger S, Khan D, Kong E, Berger A, Pollak A, Pollak DD. The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol Ther. 2015; 149: 213-226. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25562580
- Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol. 2014; 10: 643-660. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25311587
- Estes ML, McAllister AK. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci. 2015; 16: 469-486. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26189694
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009; 204: 313-321. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19136031
- Işıkay CT, Özsan H. Şizofrenide viral ve otoimmün etyoloji; literatürün gözden geçirilmesi. Ankara Üniversitesi, Tıp Fakültesi Mecmuası. 1998; 51: 101-106.
- Howes OD, McCutcheon R. Inflammation and the neural diathesis-stress hypothesis of schizophrenia: a reconceptualization. Transl Psychiatry. 2017; 7: e1024. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28170004
- Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schzophr Bull. 2013; 39: 1174-1179. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24072812
- Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, et al. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. 2015; 2: 197-199. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26359903
- Miyaoka T, Wake R, Hashioka S, Hayashida M, Oh-Nishi A, et al. Remission of Psychosis in Treatment-Resistant Schizophrenia following Bone Marrow Transplantation: A Case Report. Front Psychiatry. 2017; 8: 174. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28983259
- Aydın MŞ, Yiğit EN, Vatandaşlar E, Erdoğan E, Öztürk G. Transfer and Integration of Breast Milk Stem Cells to the Brain of Suckling Pups. Sci Rep. 2018; 8: 14289.
- Kacsoh BZ, Bozler J, Bosco G. Drosophila species learn dialects through communal living. Plos Genetics. 2018; 14: e1007430. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30024883
- Christian MA, Samms-Vaughan M, Lee M, Bressler J, Hessabi M, et al. Maternal exposures associated with autism spectrum disorder in jamaican children. JAutism Dev Disord. 2018; 48: 2766-2778. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29549549
- Glynn LM, Howland MA, Sandman CA, Davis EP, Phelan M, et al. Prenatal maternal mood patterns predict child temperament and adolescent mental health. JAffect Disord. 2018; 228: 83-90. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29241049
- Vizzini L, Popovic M, Zugna D, Vitiello B, Trevisan M, et al. Maternal anxiety, depression and sleep disorders before and during pregnancy, and preschool ADHD symptoms in the NINFEA birth cohort study. Epidemiol Psychiatric Sci. 2018; 1-11. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29665879
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010; 68: 314-319. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20674602
- Fisher J, Cabral de Mello M, Patel V, Rahman A, Tran T, et al. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bull World Health Organ. 2012; 90: 139G-149G. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22423165
- Walder DJ, Laplante DP, Sousa-Pires A, Veru F, Brunet A, et al. Prenatal maternal stress predicts autism traits in 6½ yearold children: Project Ice Storm. Psychiatry Res. 2014;219: 353-360. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24907222
- Betts KS, Williams GM, Najman JM, Alati R. The relationship between maternal depressive, anxious, and stress symptoms during pregnancy and adult offspring behavioral and emotional problems. Depress Anxiety. 2015; 32: 82-90. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24788841
- Kingsbury M, Weeks M, MacKinnon N, Evans J, Mahedy L, et al. Stressful life events during pregnancy and offspring depression: evidence from a prospective cohort study. J Am Acad Child Adolesc Psychiatry. 2016; 55: 709-716e2. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27453085
- St-Hilaire A, Steiger H, Liu A, Laplante DP, Thaler L, et al. A prospective study of effects of prenatal maternal stress on later eatingdisorder manifestations in affected offspring: preliminary indications based on the Project Ice Storm cohort. Int J Eat Disord. 2015; 48: 512-516. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25808647
- Zhu P, Hao JH, Tao RX, Huang K, Jiang XM, et al. Sex-specific and timedependent effects of prenatal stress on the early behavioral symptoms of ADHD: a longitudinal study in China. Eur Child Adolesc Psychiatry. 2015; 24: 1139-1147. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25791080
- Pickles A, Sharp H, Hellier J, Hill J. Prenatal anxiety, maternal stroking in infancy, and symptoms of emotional and behavioral disorders at 3.5 years. Eur Child Adolesc Psychiatry. 2017; 26: 325-334. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27464490
- Schepanski S, Buss C, Hanganu-Opatz IL, Arck PC. Prenatal Immune and Endocrine Modulators of Offspring’s Brain Development and Cognitive Functions Later in Life. Front Immunol. 2018; 9: 2186. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30319639
- Demirbek B, Yurt E. Can Microchimerism Find Itself a Place in Psychiatric Research? Current Approaches in Psychiatry. 2011; 3: 296-308.
- Demirhan O, Ozturk N, Aydin N, Yildizdas HY, Demirbek B, et al. Effect of fetal microchimeric cells on the development of postnatal depression. Med Clin Arch. 2019; 3: 1-6.